
We can measure small amounts of sample using a zero-background sample holder. We use a McCrone Mill to ensure homogeneous powders with a grain size smaller than 10 µm.

The grain size of the sample is perhaps the most important aspect related to random powder mount preparation. Sample preparation depends on which type of mount is required, but in both cases, sample preparation is more important than the settings of the X-ray diffractometer in determining the quality of the results. In our lab, we measure two types of mounts: (1) random powder mounts and (2) oriented clay mounts. Amorphous materials, such as glass, do not produce a diffraction pattern, but only broad scattering peaks.
XRAY DIFFRACTION ANALYSIS SOFTWARE
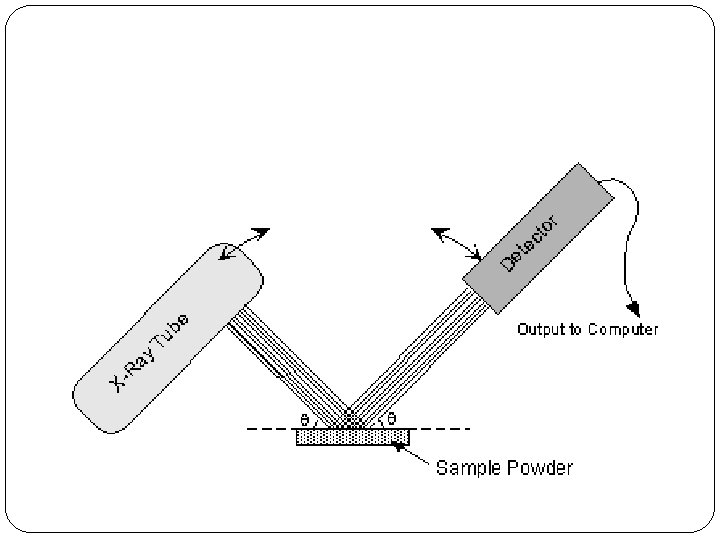
Using the software diffrac.eva (Bruker), we compare the diffraction pattern to a standard reference (PDF-4) and/or calculated patterns to identify minerals in a sample. Each mineral has a set of spacings between planes of atoms. Bragg’s Law relates the wavelength of electromagnetic radiation to the spacing between planes of atoms in a crystal lattice and the angle of diffraction. When a focused monochromatic X-ray beam, with a wavelength similar to the spacing between atoms in the crystal lattice, interacts with these planes of atoms, constructive interference takes place and part of the beam is diffracted. The three-dimensional structure of crystals is defined by regular, repeating planes of atoms that form a crystal lattice. XRD is a nondestructive analytical technique used to identify (and quantify) minerals and other crystalline materials in samples. The detector is a LYNXEYE XE-T with 192 measuring points. The instrument is a Bruker-AXS D8 ADVANCE X-ray diffractometer DAVINCI design with sample changer. The Sedimentology lab of the GeoLab has an XRD since September 2017. Contact information for prospective students.Communications and Marketing Department.Access to international research facilities.Paleomagnetism Group & Paleomagnetic Laboratory.Earth Simulation Laboratory Close submenu +.Webinar use of nanoSIMS in Life Sciences.Webinar use of nanoSIMS in Earth Sciences.Multiscale Porous Media Lab Close submenu +.Fourier-transform infrared spectroscopy.Our labs and facilities Close submenu +.Laboratories and Collaboration Close submenu +.Training for professionals Close submenu +.Compound 1 also showed comparable gas sorption capacity towards CO2 (64.5 cm3/g), N2 (72.25 cm3/g) and H2 (45.30 cm3/g) at 1 bar. The limit of detection for Fe3+, Cr3+, Al3+ ions were 75, 257 and 107 ppb, respectively. However, photoluminescence studies in the presence of other common metal ions such as Cd2+, Cu2+, Mg2+, Ca2+, Co2+, K+, Mn2+, Pb2+, Zn2+, Na+ in aqueous medium show negligible turn on effect. The compound 1 also exhibits photoluminescence based sensing behaviors towards Fe3+, Cr3+ and Al3+ ions in aqueous medium based on luminescence quenching effect. The compound 1 shows selective and efficient sorption of large anionic dye remazol brilliant blue R (RBBR) in aqueous medium, whereas compound 1 exhibits low sorption towards orange G (OG), methyl orange (MO), methylene blue (MB) and rhodamine B (RhB). The two dimensional structures are arranged in AAA….fashion to form a three dimensional packing arrangement. The single crystal X-ray studies show that connectivity among Cd2+ ions, PDA and 2,4,5-tri-4-pyridyl-1H-imidazole ligands form two-dimensional structure. A Cd(II)-based metal–organic framework (MOF) (PDA = 1,4-phenylenediacetate and L = 2,4,5-tri-4-pyridyl-1H-imidazole), 1, has been synthesized by solvothermal method and characterized by single-crystal X-ray diffraction techniques, powder X-ray diffraction, Fourier Transform Infrared (FTIR) and thermogravimetric analysis (TGA).


 0 kommentar(er)
0 kommentar(er)
